Related Articles

The Hidden Danger: Unraveling the Complex Link Between Talc, Asbestos, and Cancer

The Hidden Sleep Divide: Why Women May Need More Rest Than Men




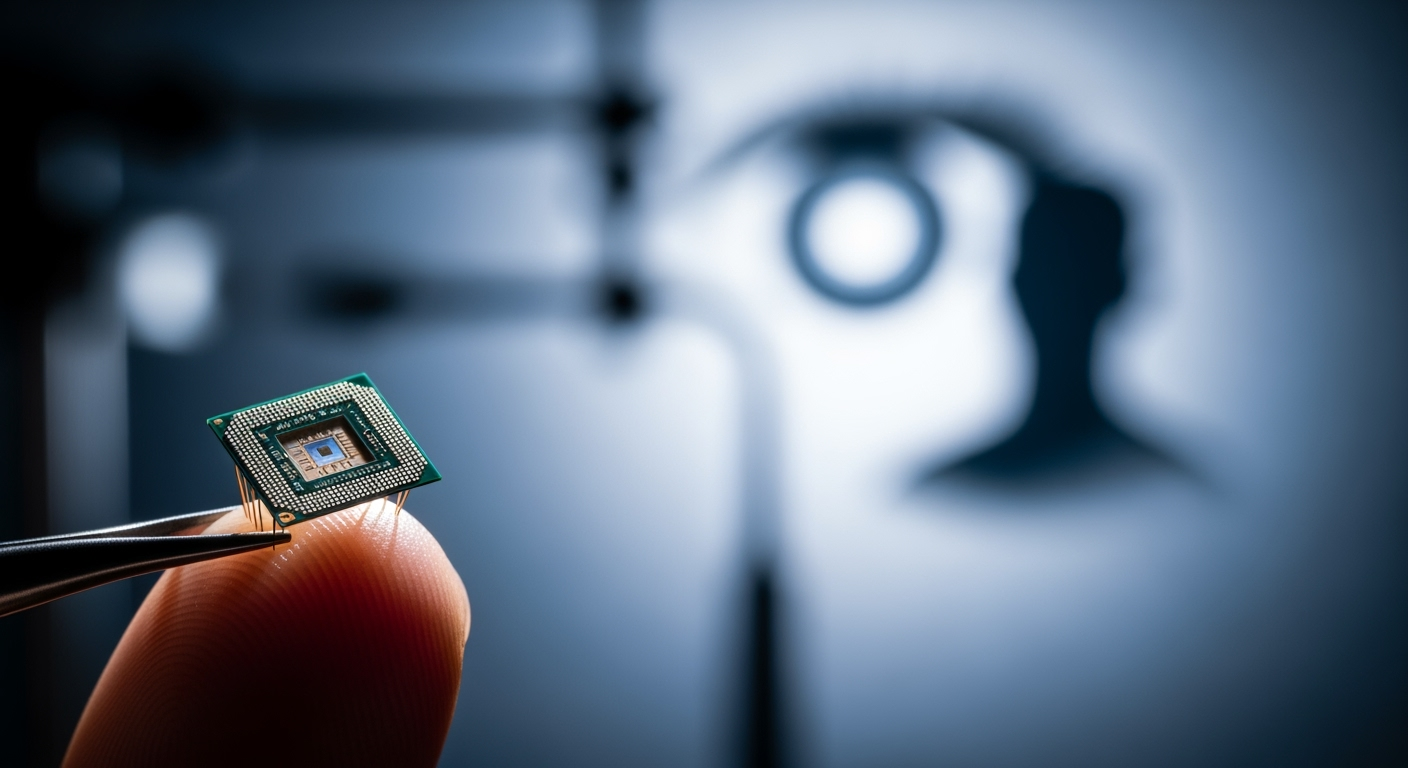
A groundbreaking new subretinal implant, often described as a "bionic eye," is revolutionizing the landscape of vision restoration for individuals suffering from severe sight loss, particularly those impacted by dry age-related macular degeneration (AMD) with geographic atrophy (GA). Recent clinical trials have demonstrated that this tiny, innovative chip, paired with specialized augmented-reality glasses, can restore functional central vision, including the ability to read letters, numbers, and words—a feat previously unattainable for many patients. This medical advancement offers a profound shift from merely slowing disease progression to actively regaining lost visual capabilities, instilling renewed hope and independence for millions worldwide.
At the heart of this medical marvel is the PRIMA System, a sophisticated neurostimulation device developed by Science Corporation, with foundational work led by Professor Daniel Palanker at Stanford University. The system comprises three main components: a miniature, wireless retinal implant, a pair of augmented-reality glasses equipped with a camera and digital projector, and a compact pocket processor. The implant itself is remarkably small, measuring just 2mm by 2mm and a mere 30 micrometers thick—approximately half the thickness of a human hair. This ultra-thin microchip, which functions like a tiny solar panel with 378 photovoltaic cells, is surgically inserted beneath the retina in the area where light-sensing cells have deteriorated.
The operational principle of the PRIMA System is both elegant and effective. The augmented-reality glasses capture images from the environment and transmit them as a beam of invisible near-infrared light to the implant. Crucially, this infrared light is imperceptible to the human eye, ensuring it does not interfere with any remaining peripheral vision. The subretinal implant then absorbs this light, converting it into electrical signals. These electrical impulses are then sent to the healthy nerve cells in the retina (specifically the bipolar cells) that would normally relay visual information to the brain, effectively bypassing the damaged photoreceptors. An artificial intelligence algorithm within the pocket processor refines this information, allowing the glasses to focus on key objects in the visual field. This direct stimulation allows the brain to interpret these patterns as visual input, initiating a process where patients learn to "see" again.
The efficacy of the PRIMA System has been rigorously evaluated through multi-center clinical trials conducted across Europe and the United States, involving institutions such as Moorfields Eye Hospital in London, the University of Bonn, and Stanford Medicine. The results, published in The New England Journal of Medicine, have been overwhelmingly positive. In a significant study involving 38 participants with dry AMD, 84.4% of those on the trial regained the ability to read letters, numbers, and words using their implanted eye. Of the 32 participants who completed 12 months of follow-up, 26 achieved clinically meaningful improvements in visual acuity. Patients, many of whom had no central vision prior to the implant, demonstrated an average improvement of reading up to five lines on a standard eye chart. Some individuals even improved by as many as 10 lines, with some achieving vision equivalent to 20/42 with digital enhancements like zoom and contrast adjustment.
The human impact of this technology is profound. Patients have reported a significant improvement in their quality of life, regaining the ability to perform daily tasks that require central vision. For instance, one participant, Sheila Irvine, an avid reader who described her vision before the implant as "having two black discs in my eyes," found renewed optimism and could again read text on tins and engage in crosswords. Another patient from France used the device to navigate the Paris Metro, highlighting the system's capacity to aid in complex tasks beyond simple reading. This restoration of functional central vision for those with geographic atrophy, a condition for which no other restorative treatment exists, marks a monumental step forward in ophthalmology.
While the PRIMA System represents a cutting-edge advancement, it builds upon earlier innovations in visual prostheses. The Argus II Retinal Prosthesis System, for example, has been instrumental in offering partial vision to patients with advanced retinitis pigmentosa (RP). Unlike PRIMA, which targets dry AMD by replacing photoreceptor function, the Argus II is an epiretinal implant that stimulates existing retinal cells to produce light perception and rudimentary form vision. FDA-approved as a Humanitarian Use Device, the Argus II has enabled patients to perceive light and motion, and in some cases, recognize large-print text, with long-term studies showing improved visual function and quality of life for up to five years.
The advent of the PRIMA System signifies a new era, moving beyond light perception to offer more sophisticated and functional central vision restoration, particularly for the estimated five million people globally affected by geographic atrophy. The ability to read letters and words provides a level of independence and engagement with the world that was previously considered lost. However, it is important to note that patients undergoing PRIMA implantation require extensive rehabilitation and training to learn how to interpret the new visual signals. The brain must adapt to integrating the prosthetic vision with any remaining peripheral vision.
Despite its remarkable successes, the PRIMA System, like any emerging technology, presents certain considerations and ongoing developmental paths. The vision restored by PRIMA is currently monochromatic (black-and-white) and does not fully replicate natural, detailed sight. Patients must also adapt to wearing the augmented-reality glasses and pocket processor, which are integral to the system's operation. While generally safe, adverse effects—typical of eye surgery—were reported in a minority of participants, though most were resolved quickly. Furthermore, the training period required to interpret the new visual signals can be extensive, demanding patience and dedication from patients.
Looking ahead, researchers and developers are optimistic about expanding the capabilities and applications of this technology. Science Corporation has already submitted an application for CE Mark approval in Europe, a critical step toward wider availability for patients. Future research aims to further improve visual acuity, potentially allowing for finer details and even features like facial recognition. The potential for adapting this technology to treat other forms of vision loss beyond dry AMD is also a significant area of exploration. The ongoing evolution of retinal prostheses promises a future where the impact of previously untreatable blindness can be significantly mitigated, continuously expanding the horizons of restored sight.
The introduction of tiny retinal implants like the PRIMA System marks a pivotal moment in medical science, ushering in an era where the dream of restoring functional vision for those with profound sight loss is increasingly becoming a reality. For millions living with conditions like dry age-related macular degeneration, this technology offers more than just improved perception; it delivers the tangible hope of regaining a connection to the visual world, fostering greater independence, and significantly enhancing their quality of life. As research progresses and the technology refines, the narrative of untreatable blindness is steadily being rewritten, piece by tiny chip by tiny chip.